If your business is into pharmaceutical/nutraceutical industry, you must be aware of the importance of batch serialization. Additionally, the challenges faced from being able to trace inventory by batch number to managing serialized items individually. It is often time-consuming and extremely inconvenient.
In this situation, you must be looking for a tool that supports new technology and innovation. As well as meets FDA requirements and serializes finished products.
While the existing monolithic ERP systems barely suffice in meeting all these requirements, you are in dire need of next-gen ERP systems which are equipped with revolutionary technologies to help meet future needs.
To track and trace batch or lot numbers and serial numbers efficiently, we at Consultare recommend a process manufacturing plug-in on your existing SAP Business One.
The plug-in is a comprehensive solution that covers the main requirements of manufacturing ERP.
Our support is twofold.
1. Serialization
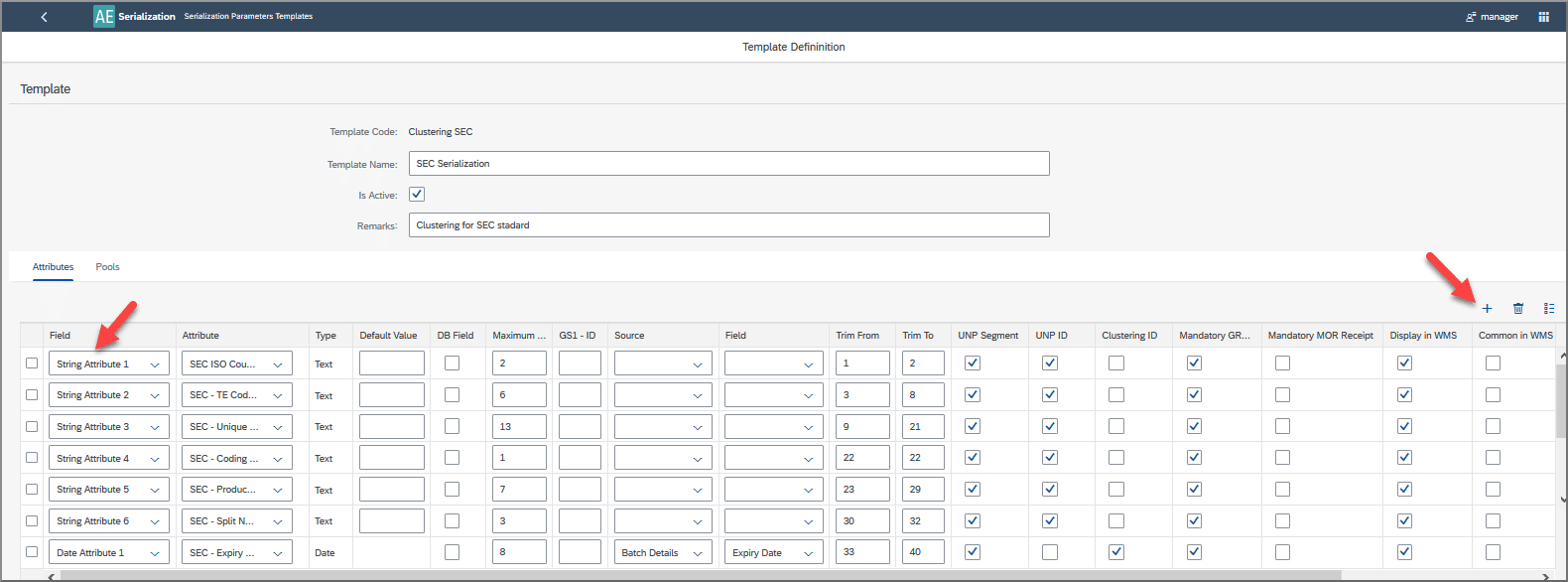
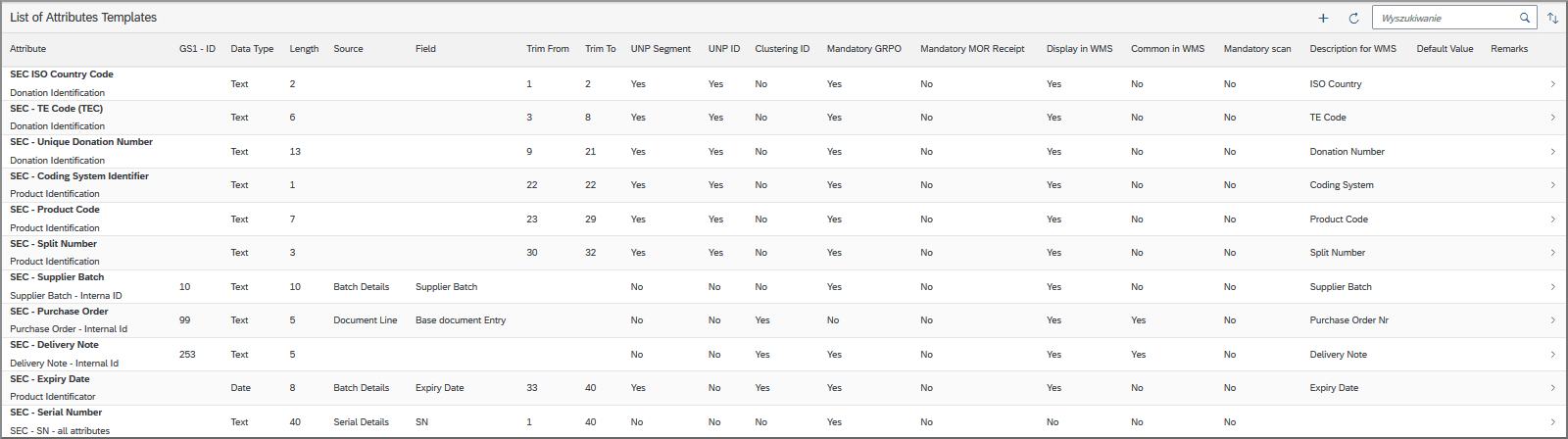
This plugin allows users to assign serial numbers to batches based on highly customizable rules in a process called “clustering”, and to later reorganize serialized batches if required.
Clustering using the serialization plugin – How to Use?
- Using the Serialization plugin, you can use data to build batches from your serialized items according to your individual requirements.
- You can set up templates to automatically generate serial numbers that draw on characters from attributes such as expiry date, manufacture date, product code and so on.
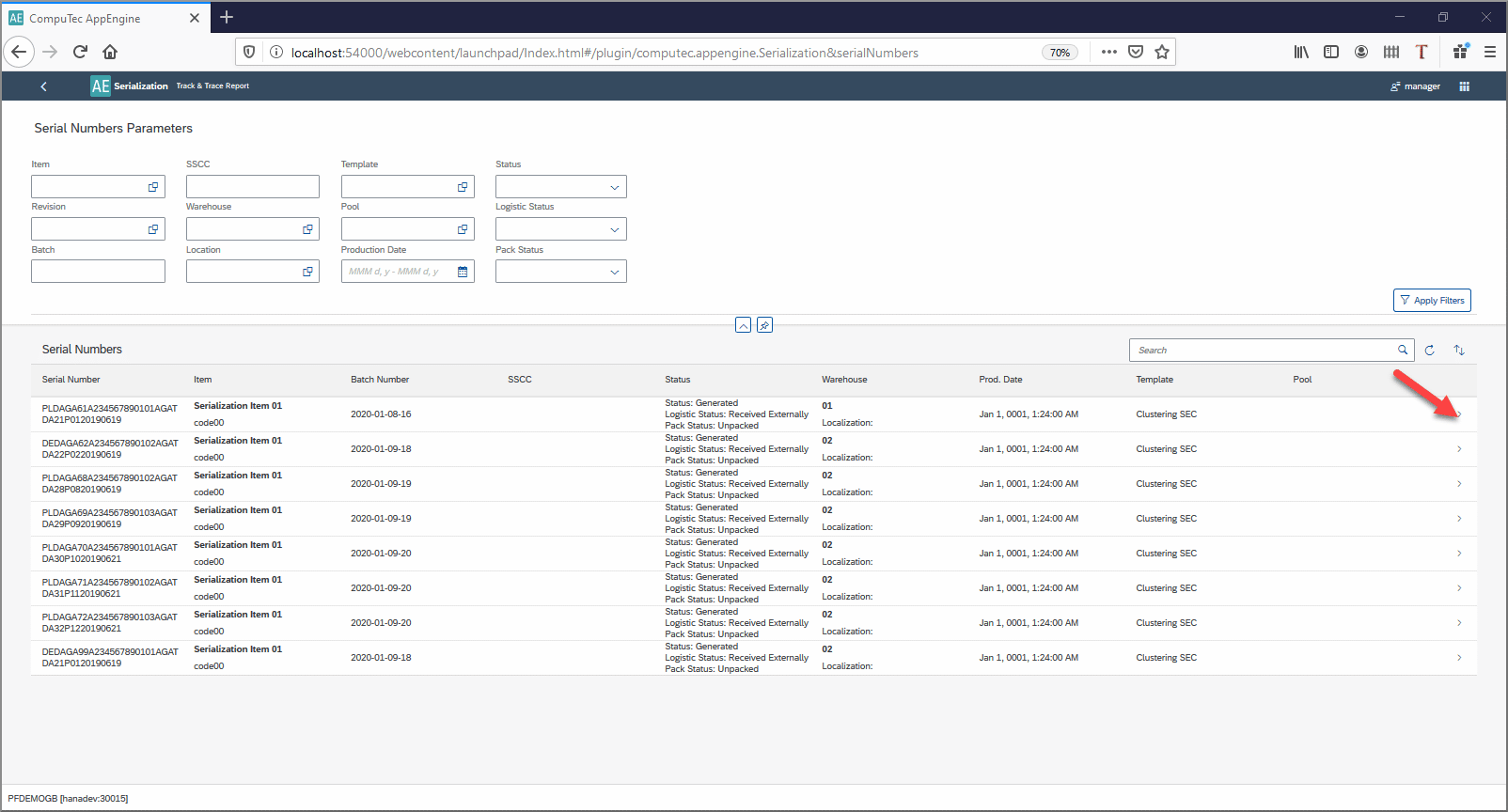
A value-add – The track and Trace support:
This feature in the Serialization plugin allows you to trace the history of any serial number including which batches it has been in until now.
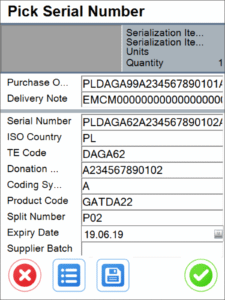
2. Support for serialization in WMS
To allow warehouse operatives to pack and repack the serialized inventory correctly with the aid of barcode scanners and to record a variety of transactions for serialized batches.
Our WMS supports Serialization for quite several transactions. Currently, these comprise of Goods Receipt, Goods Receipt PO, Stock Transfer, Goods Issue, Delivery and Storage Unit operations.
Do you want to protect your business and meet country-specific requirements for drug serialization?